Antisense Oligonucleotide Therapeutics Clinical Trial Pipeline Appears Robust With 70+ Key Pharma Companies Actively Working in the Domain | DelveInsight
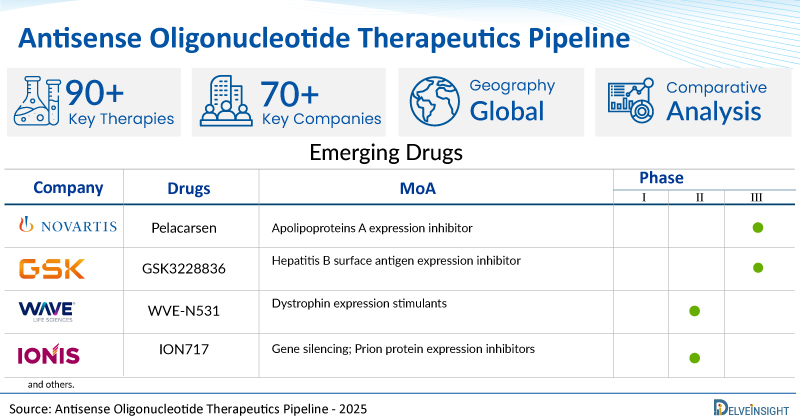
New York, USA, Aug. 28, 2025 (GLOBE NEWSWIRE) -- Antisense Oligonucleotide Therapeutics Clinical Trial Pipeline Appears Robust With 70+ Key Pharma Companies Actively Working in the Domain | DelveInsightAntisense oligonucleotides (ASOs) are gaining momentum as a promising therapeutic approach, particularly for rare genetic and neuromuscular disorders. Their ability to selectively modulate gene expression at the mRNA level offers targeted treatment options where conventional therapies fall short. The increasing number of FDA-approved ASO drugs and ongoing clinical advancements reflect strong innovation potential in this space.DelveInsight's 'Antisense Oligonucleotide Therapeutics Pipeline Insight 2025' report provides comprehensive global coverage of pipeline antisense oligonucleotide therapeutics in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the antisense oligonucleotide therapeutics domain.Key Takeaways from the Antisense Oligonucleotide Therapeutics Pipeline ReportDelveInsight's antisense oligonucleotide therapeutics pipeline report depicts a robust space with 70+ active players working to develop 90+ pipeline antisense oligonucleotide therapeutics. Key antisense oligonucleotide therapeutics companies such as Novartis Pharmaceuticals, GSK, Ionis Pharmaceuticals, Wave Life Sciences, Vanda Pharmaceuticals, NS Pharma, TransCode Therapeutics, Amylyx Pharmaceuticals, Inc., Biogen, Cure Rare Disease, Cardior Pharmaceuticals, Bio-Path Holdings, Sunhawk Vision Biotech, Isarna Therapeutics, Ausper Biopharma Co., Ltd, Lipigon Pharmaceuticals, Dyne Therapeutics, Flamingo Therapeutics, Vico Therapeutics, Laboratoires Théa, and others are evaluating new antisense oligonucleotide therapeutics to improve the treatment landscape.Promising clinical trial antisense oligonucleotide therapeutics, such as Pelacarsen, GSK3228836, WVE-N531, ION717, BP1002, NS-051/NCNP-04, TTX MC 138, AMX0114, BIIB080, Salanersen, CRD002, CDR132L, BP1001, SHJ002, ISTH0036, VGT-1849A, AHB-137, Lipisense, DYNE-101, Danvatirsen, VO 659, Ultevursen, and others, are in different phases of antisense oligonucleotide therapeutics clinical trials.In July 2025, Biogen and Stoke Therapeutics announced the presentation of data from an analysis that informed the design of the Phase III EMPEROR study and evaluated the potential effects of the Phase III zorevunersen dosing regimen. The data are complementary to previously reported data from a broader cohort of patients treated with zorevunersen in the Phase I/IIa and open label extension (OLE) studies that showed improvements within the first 9 months and continuing improvements through an additional two years.In June 2025, Amylyx Pharmaceuticals announced that the US Food and Drug Administration (FDA) had granted Fast Track designation to AMX0114, an investigational antisense oligonucleotide (ASO) targeting calpain-2 for the treatment of people living with amyotrophic lateral sclerosis.In June 2025, Ionis Pharmaceuticals announced that its partner, Biogen, shared positive topline results from the Phase I study of salanersen (ION306/BIIB115), an investigational antisense oligonucleotide (ASO) being developed for the potential treatment of spinal muscular atrophy.In May 2025, Cure Rare Disease (CRD) announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to its investigational anti-sense Oligonucleotide therapeutic for the treatment of Spinocerebellar Ataxia (SCA), including Spinocerebellar Ataxia Type 3.In April 2025, Biogen announced that the US Food and Drug Administration (FDA) had granted Fast Track designation to BIIB080, an investigational antisense oligonucleotide (ASO) therapy targeting tau, for the treatment of Alzheimer's disease.In February 2025, AusperBio, a clinical-stage biotechnology company, announced recent progress in the ongoing clinical development of its lead candidate AHB-137, an antisense oligonucleotide (ASO) therapeutic for the functional cure of chronic Hepatitis B (CHB).In January 2025, Dyne Therapeutics, Inc. announced that the US Food and Drug Administration (FDA) had granted Fast Track designation for DYNE-101 for the treatment of myotonic dystrophy type 1 (DM1).In January 2025, Ionis partner Novartis shared in their year-end earnings report that Phase III pelacarsen data is now expected in the first half of 2026, with subsequent regulatory submissions in the second half of next year. This update is based on the accrual rate of blinded events in the ongoing Phase 3 Lp(a)HORIZON event-driven cardiovascular outcomes study that Novartis is conducting in more than 8,000 patients.Request a sample and discover the recent advances in antisense oligonucleotide therapeutics @ Antisense Oligonucleotide Therapeutics Pipeline ReportThe antisense oligonucleotide therapeutics pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage antisense oligonucleotide therapeutics, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the antisense oligonucleotide therapeutics clinical trial landscape. Antisense Oligonucleotide Therapeutics OverviewAntisense oligonucleotide (ASO) therapeutics are short, synthetic strands of nucleic acids, typically 15–25 bases long, designed to bind specifically to messenger RNA (mRNA) and regulate gene expression. By targeting complementary RNA sequences, ASOs can interfere with the production of disease-related proteins through mechanisms such as inhibiting translation, modifying splicing patterns, or promoting mRNA degradation.Structurally, ASOs are composed of nucleotide bases and a sugar-phosphate backbone, which is often chemically modified to enhance stability and resistance to enzymatic breakdown. Common modifications include 2'-O-methyl, 2'-MOE, phosphorothioate linkages, and more advanced structures like locked nucleic acids (LNA) and constrained ethyl (cEt). These chemical enhancements increase binding affinity, specificity, and tissue penetration while improving safety and pharmacokinetics. ASOs function through several key mechanisms:RNase H-mediated degradation, where the ASO binds to mRNA to form a DNA-RNA hybrid that is then cleaved by RNase H.Steric blocking, where the ASO physically obstructs ribosomal or splicing machinery.Splice modulation, where ASOs direct the inclusion or exclusion of specific exons during pre-mRNA processing, offers therapeutic benefits in various genetic disorders.Thanks to their precise targeting capabilities, ASOs have become a powerful modality for treating rare, genetic, neuromuscular, and neurodegenerative diseases, especially those with limited therapeutic options. Their high specificity allows them to address previously "undruggable" targets, enabling both individualized and broader therapeutic approaches. With several ASO-based treatments already approved by regulatory agencies and continued innovation in delivery and chemistry, ASOs represent a rapidly evolving and promising class of genetic medicines.Find out more about antisense oligonucleotide therapeutics @ Full story available on Benzinga.com









